Advantages, Disadvantages, and Applications
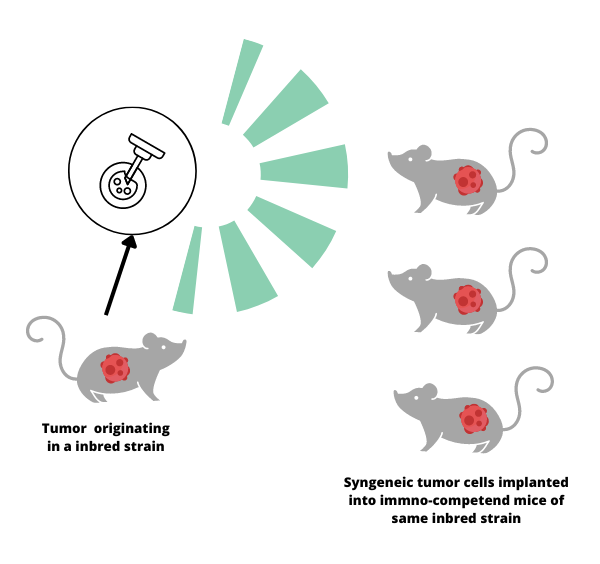
Syngeneic mouse models play a crucial role in cancer research; they help aid in the study of how the immune system interacts with cancer cells. A syngeneic mouse model is created by transplanting cancer cells from one mouse to another that is genetically identical, or from the same strain. This allows researchers to observe how the immune system responds to the tumor in a realistic and controlled environment.
The history of syngeneic mouse models dates back to the 1940s when scientists started using inbred mouse strains to study cancer. This genetic uniformity made it possible for researchers to create syngeneic models by transplanting cancer cells from one mouse to another without the risk of rejection. As a result, they could observe how the immune system fought against cancer without interference from other factors.
Over time, the development of these models has advanced significantly, leading to a more accurate representation of the complex interactions between the immune system and cancer cells. In recent years, researchers have combined syngeneic models with cutting-edge genetic engineering techniques to create mice with specific genetic modifications. This has opened up studies of the effects of particular genes on cancer development and the immune response.
State-of-the-art syngeneic mouse models are particularly valuable for studying immunotherapy treatments that are designed to boost the immune system's ability to target and destroy cancer cells. Researchers are gaining insights into how these therapies interact with the immune system, helping to improve their effectiveness and minimize potential side effects.
The Benefits of Syngeneic Models
Unlike immunodeficient or immunocompromised models, syngeneic models have a fully functional immune system. This enables researchers to study the complex interactions between the immune system and tumor cells, and to investigate the effects of immunotherapy treatments in a more biologically relevant context.
Furthermore, syngeneic models allow for the study of the tumor microenvironment, which consists of various cell types, extracellular matrix components, and signaling molecules that play a critical role in tumor growth, progression, and response to therapy. And, although syngeneic models are genetically similar, they still exhibit some level of genetic variability, which can provide insights into the role of genetics in cancer susceptibility, progression, and treatment response.
The Limitations of Syngeneic Models
Though the development of syngeneic studies was a major advancement in biomedical research — one which is still highly relevant in many lines of cancer research today — they are not ideal for all situations.
Limited representation of human cancers
Syngeneic models involve murine (mouse-derived) tumor cells, which may not fully represent the complexity and diversity of human cancers. They may lack specific genetic mutations and molecular features found in human tumors, limiting the translatability of research findings to human patients.
Immune system differences
While syngeneic models have a fully functional immune system, it is a mouse immune system, which may not perfectly mimic human immune responses. Consequently, the effectiveness of immunotherapies and the interactions between the immune system and tumor cells may differ between mice and humans.
Lack of genetic diversity
Syngeneic models use inbred mouse strains, which have limited genetic diversity compared to the human population. This reduces the ability to study the impact of genetic variations on cancer development, progression, and treatment response.
Tumor implantation
The process of implanting tumor cells into host mice may not always accurately represent the natural process of tumor development and growth. Spontaneous tumor formation, as seen in genetically engineered mouse models, may more closely mimic the natural course of human cancers.
Syngeneic vs Transgenic Models
Unlike syngeneic models, which are created by taking cells or tissues from a donor mouse and implanting them into a genetically identical recipient mouse, transgenic models are created by altering a mouse’s genome (such as by introducing foreign DNA), essentially creating a new, genetically modified organism. Transgenic mice have specific genes either added or removed, allowing scientists to study the effects of those specific genetic changes on the development and progression of diseases.
Transgenic models are optimal for studying a wide range of diseases, including genetic disorders, neurodegenerative diseases, and cardiovascular diseases. They can also help researchers understand the function of specific genes in normal development and disease progression.
While both syngeneic and transgenic models have their merits, they are not equally suited for all research situations. For example, syngeneic models may not be well suited for studying the role of genetic diversity in disease development, as their identical genetic makeup limits the range of genetic factors that can be examined. Similarly, transgenic models might not be the best choice for studies focusing on the immune system, as the introduction of foreign DNA can sometimes lead to unexpected immune responses, complicating the interpretation of results.
Syngeneic vs Humanized Mice
Syngeneic mice are somewhat like identical twins (though not naturally occurring) and their duplicate genetic makeup limits the range of factors to study. By contrast, humanized mice are genetically engineered to carry human genes, cells, or tissues. This modification allows the mice to more closely mimic human biological processes, making them an invaluable tool for studying human diseases and evaluating the safety and efficacy of potential treatments.
Humanized mice are especially useful for researching conditions that have a strong human-specific component, such as infectious diseases caused by human-specific pathogens, cancer, and autoimmune diseases. They also play a vital role in testing the safety and effectiveness of human-specific drugs and therapies. However, humanized mice may not be well-suited for studying certain aspects of mouse biology or diseases that have a strong species-specific component since they are designed to mimic human biology.
LIDE delivers innovations in oncology translational research and immuno-oncology. Contact Global Vice President, Josh Caggiula today to learn more about our first-class research capabilities.