LIDE provides in vivo efficacy evaluation of various CAR-T therapies in either CDX or PDX models.
 |
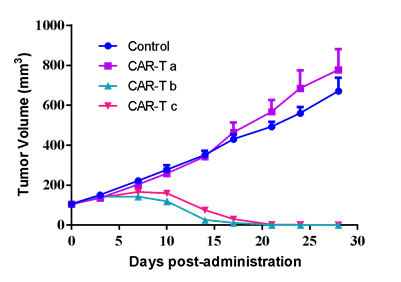 |
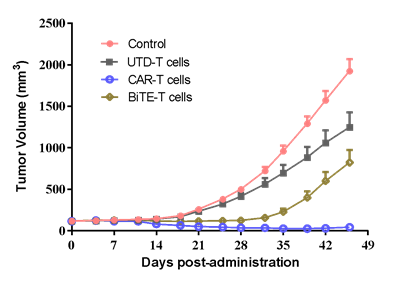
| SK-OV-3 human ovarian cancer cells were inoculated into the right flank in NCG mice. Mice were randomized when average tumor volume grown up to ~120 mm3 and dosing were initiated accordingly. Varied T cells were dosed at *107 level through i.v. for 2 injections at Day0 and Day3, while tumor volume and body weight were measured twice a week. | #LD1-0032-200670 human ovarian PDX model were inoculated into the right flank in NCG mice. Mice were randomized when average tumor volume grown up to ~110 mm3 and dosing were initiated accordingly. Varied CAR-T cells were dosed once at Day0, while tumor volume and body weight were measured twice a week. |
Bio-markers determination can also be included during or at the end point of studies using mice whole blood or tumor tissues via FACS analysis.
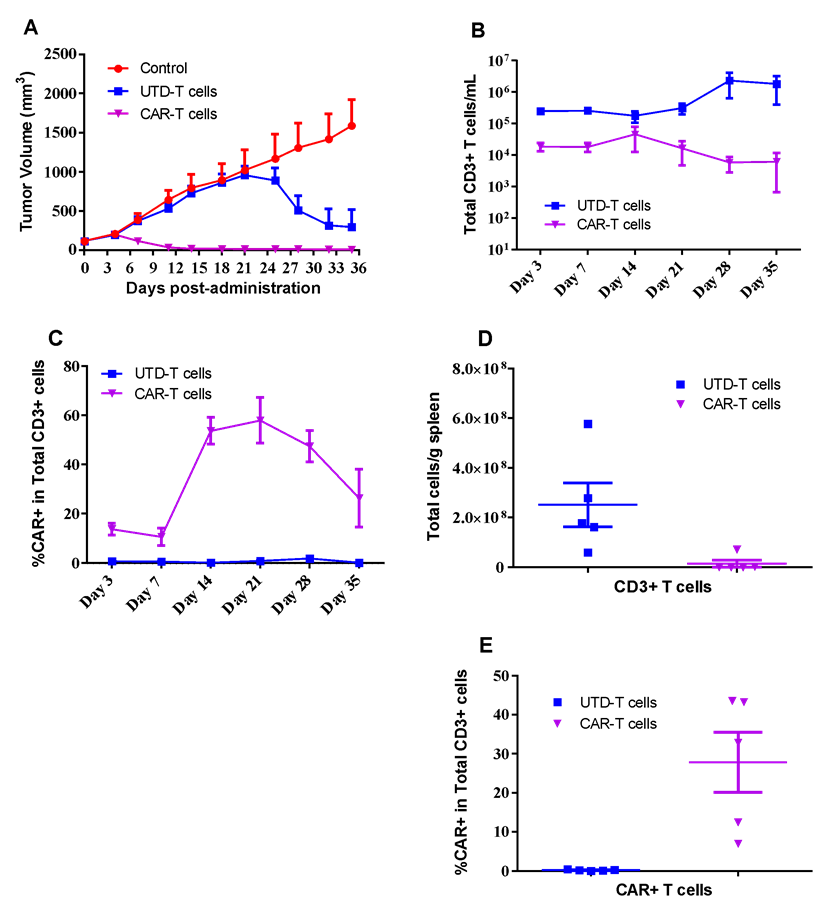
SUN620 human gastric cancer cells were inoculated into the right flank in NCG mice. Mice were randomized when average tumor volume grown up to ~110 mm3 and dosing were initiated accordingly. UTD-T or CAR-T cells were dosed at *107 level through i.v. once at Day0, while tumor volume and body weight were measured twice a week (A). Peripheral blood of each mouse were collected at indicated days after CAR-T injection, and analyzed using FACS for population of CD3+ T cells (B) or CAR+ T cells (C) determination. Spleen were collected at the end of the study, while CD3+ T cells (D) or CAR+ T cells (E) were determined via FACS assay.