LIDE has independently validated and extended how conditionally reprogrammed (CR) cells, originally developed at Georgetown, can be used in precision oncology therapy and drug R&D. CR cells allow for rapid and efficient expansion of cell cultures from patient tissue samples while maintaining the heterogeneity of the original cancer. These new cell lines provide a much better starting point than traditional immortalized cell lines in compound screens or efficacy studies.
CR Cells in Drug R&D:
- In vitro TCA compound screening
- Potential indication screening via MiniPDX assay.
- In vivo efficacy study using CRC-generated CDX.
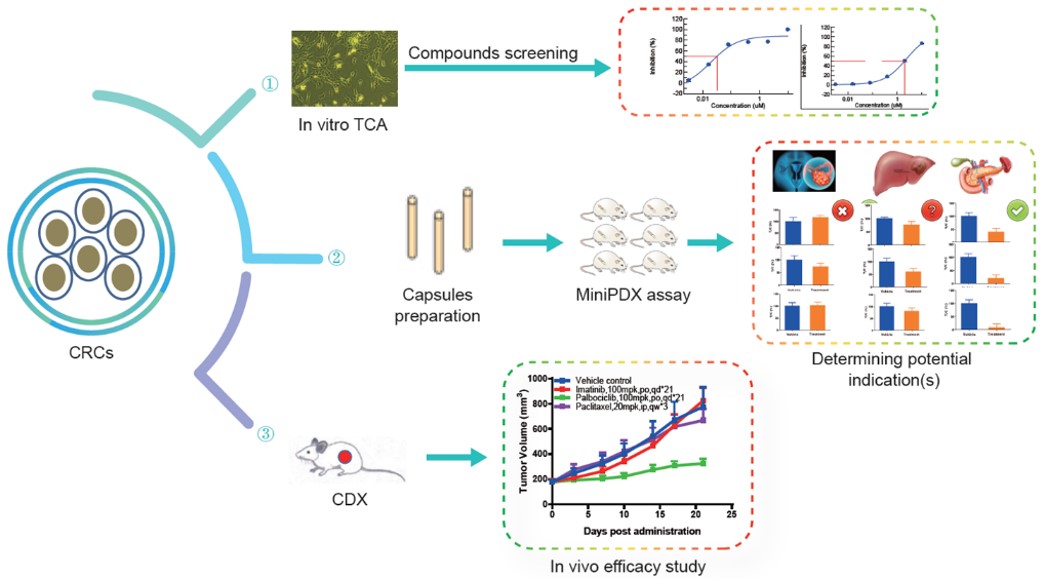
Fig. Schematic of testing options enabled by CR (conditionally reprogrammed) cells.
LIDE has developed over 100 CR cells (left) in which some of PDX matching CRCs exert special drug resistance/genetic alteration (right).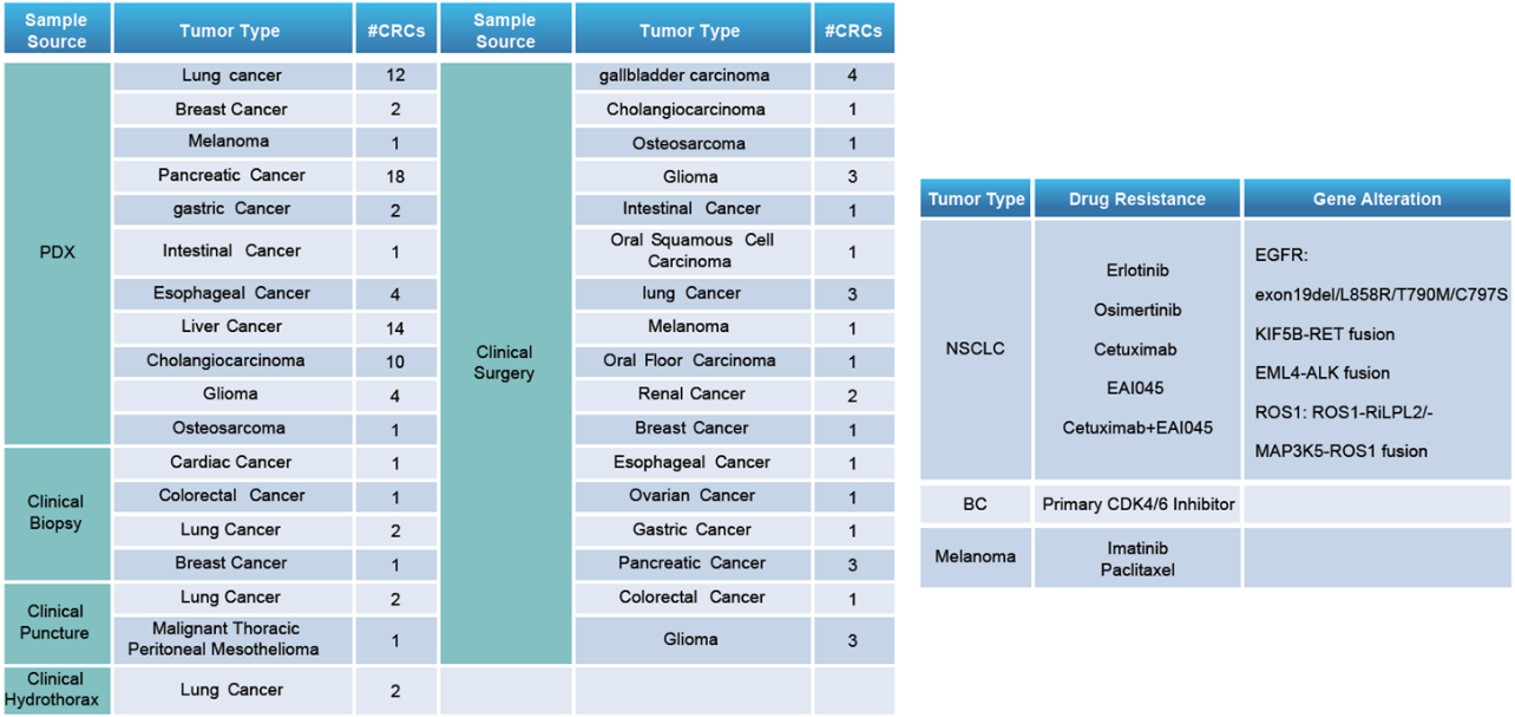
Download the full detailed list here
CR Methodology:
Originally developed at Georgetown University Medical Center, conditionally reprogrammed (CR) cells offer a novel approach to culturing cancer cells long-term, without altering the genetic background of the primary cells. This contrasts with immortalized tumor cell lines that have changed over time and do not resemble natural cancer cells anymore. Before conditionally reprogrammed cells, it was demonstrated that no more than 5% of primary tumors can be expanded in vitro over multiple generations.
The CR process leverages two different substances (a Rho kinase (ROCK) inhibitor and fibroblast feeder cells) to morph cancer cells into stem-like cells - allowing amplification without risk of genetic alterations. Conditional reprogramming enables generation of cell lines from almost 90% of tissue specimens from human normal and tumor origins, while maintaining both intratumor and intertumor heterogeneity. (Acta Pharm Sin B. 2020 Aug; 10(8): 1360-1381.) LIDE has independently validated this heterogeneity.
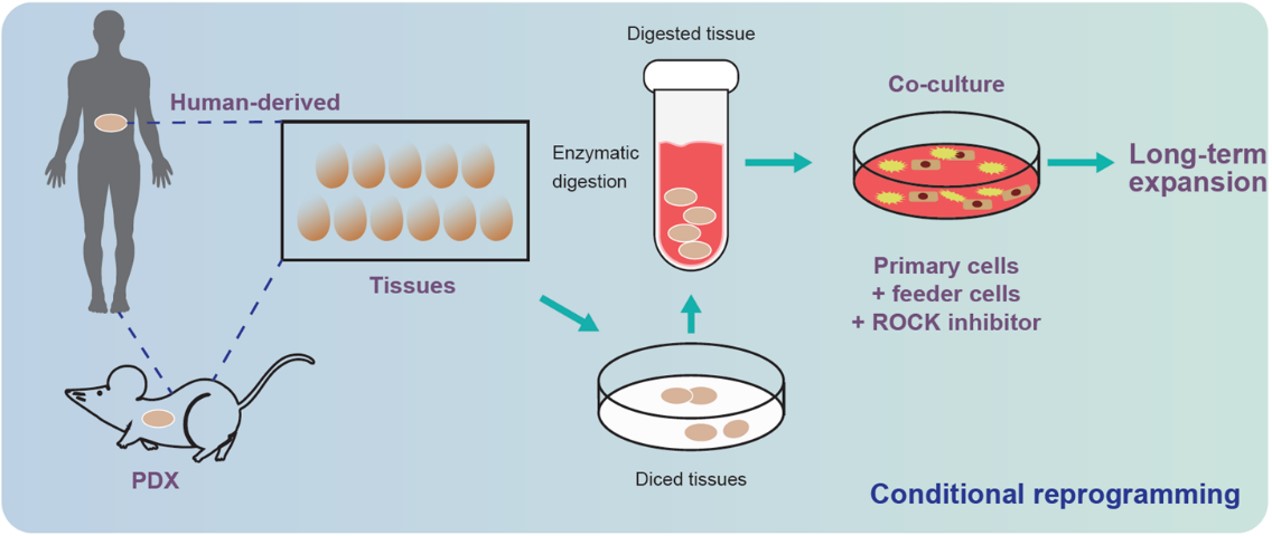
Fig. Schematic of process for conditional reprogramming cells