LIDE provides in vivo efficacy evaluation of oncolytic virus therapies in multiple models, including murine/humanized syngeneic models, CDX, PDX, or CDX/PDX with mice humanized.
 |
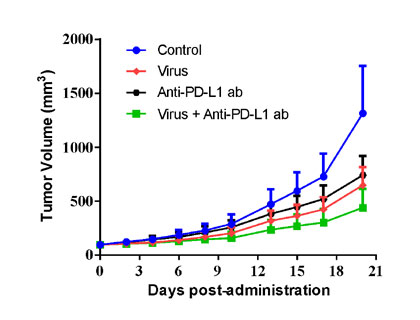 |
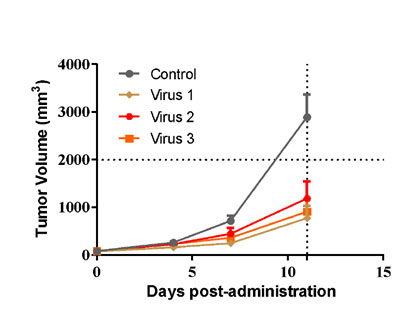
| B16-F10 murine melanoma cells were inoculated into the right flank in C57BL/6n mice. Mice were randomized when average tumor volume grown up to ~90 mm3 and dosing were initiated accordingly. Varied oncolytic virus were dosed at *108 pfu/mouse through intratumorally every other day for 3 injections, while tumor volume and body weight were measured twice a week. | MC38 murine colon cancer cells were overexpressed with TAA1 (MC38-TAA1) and were inoculated into the right flank in huPD-L1 transgenic mice. Mice were randomized when average tumor volume grown up to ~90 mm3 and dosing were initiated accordingly. Oncolytic virus at *109 pfu/mouse through intratumorally for 3 injections at Day0, Day2, and Day4, and anti-PD-L1 ab at 3 mg/kg via i.p. bi-weekly for 9 injections were administrated in either monotherapy or combination way, while tumor volume and body weight were measured twice a week. |
 |
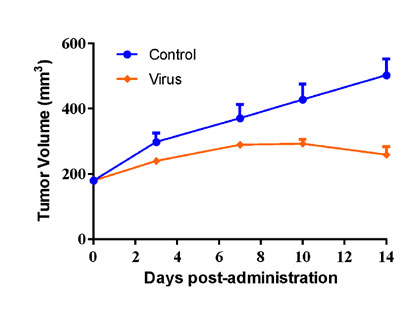 |
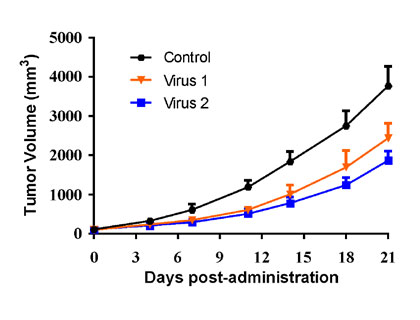
| Huh7 human Hepatocarcinoma cells were inoculated into the right flank in Balb/c nude mice. Mice were randomized when average tumor volume grown up to ~100 mm3 and dosing were initiated accordingly. Varied oncolytic virus were dosed at *1010 pfu/mouse through intratumorally every other day for 10 injections, while tumor volume and body weight were measured twice a week. | PC-3 human prostate cancer cells were inoculated into the right flank in NSG mice, while the mice were reconstituted with huPBMC via i.v. injection when average tumor volume grown up to ~100 mm3. Mice were randomized 3 days after huPBMC reconstitution and dosing were initiated accordingly. Oncolytic virus were dosed at *105 pfu/mouse through intratumorally on Day0 and Day7 post-grouping, while tumor volume and body weight were measured twice a week. |
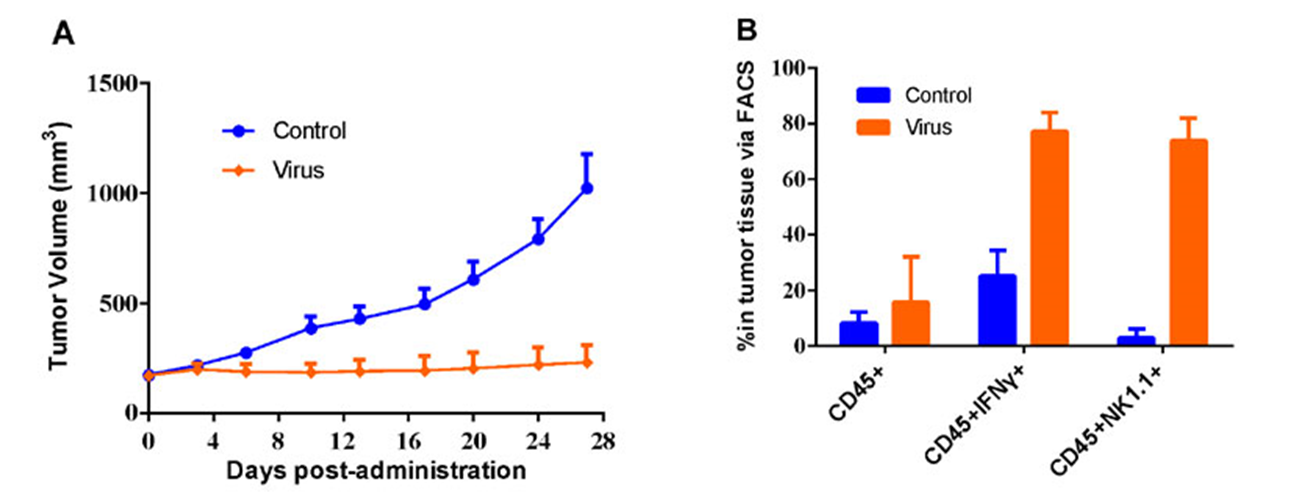
#LD1-0025-200717 human NSCLC PDX model were inoculated into the right flank in NU/NU mice. Mice were randomized when average tumor volume grown up to ~200 mm3 and dosing were initiated accordingly. Oncolytic virus were dosed through intratumorally every other day for 5 injections, while tumor volume and body weight were measured twice a week (A). Indicated markers were analyzed via FACS using tumor tissues generated at the end of the study (B).
Oncolytic virus in vitro assay
LIDE can also provide in vitro assays for oncolytic virus, such as cytokine release determination as follows:
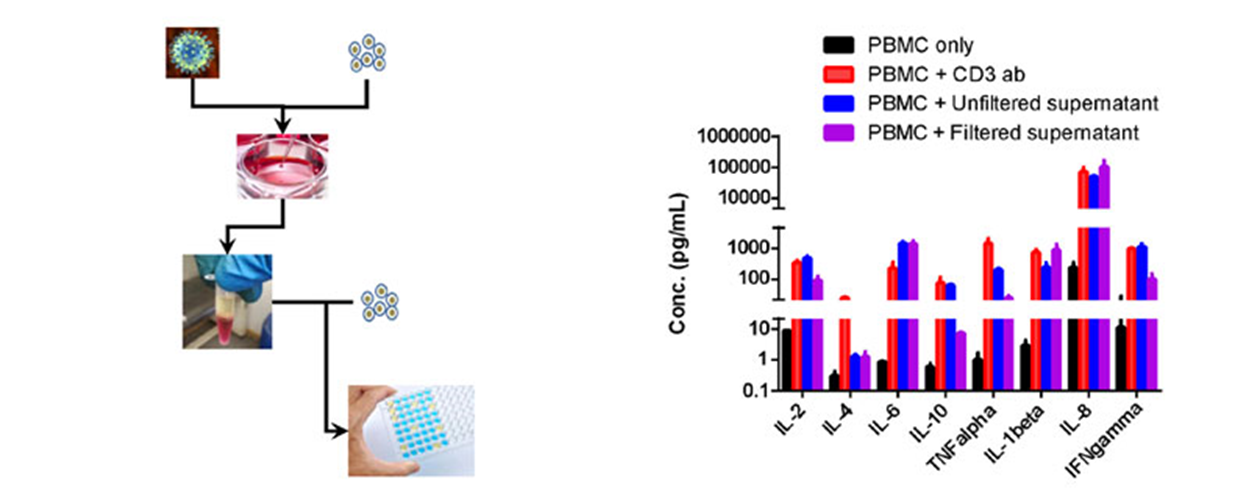
Oncolytic virus were co-incubated with cancer cells, while supernatant were collected. Then the filtered or unfiltered supernatant were added into culturing huPBMC, and cytokine release were determined using ELISA assay. (A) flowchart of the in vitro assay; (B) cytokine determined by ELISA.