The MiniPDX® test can provide drug sensitivity results in as little as 7 days vs. the 4-6 months needed for traditional PDX results. Given the time savings of MiniPDX® vs traditional PDX, it is natural to ask how results correlate between the two model types. In a published study, LIDE used 26 PDX models generated from patient tumor samples, including 14 gastric cancers, 10 lung cancers and 2 pancreatic cancers to demonstrate that drug responses in the PDX tumor graft assays correlated well with those in the corresponding MiniPDX assays. The positive predictive value of MiniPDX was 92% vs. PDX, and the negative predictive value was 81% with a sensitivity of 80% and a specificity of 93%.
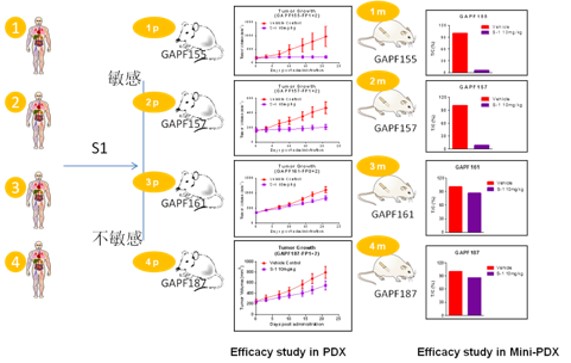
Fig. Comparing efficacy results of drug S1 in PDX assays vs. equivalent MiniPDX assays.
PDX is well studied and shown to be 89% correlated with clinical result. Taken together with MiniPDX® results, this means MiniPDX® is 82% correlated with clinical results, by calculation. In China, LIDE has verified this in the clinical setting, with over 2600 treated cases at over 80% effectiveness.
Several papers have been published supporting MiniPDX®:
- https://cancercommun.biomedcentral.com/articles/10.1186/s40880-018-0329-5
- https://cancercommun.biomedcentral.com/articles/10.1186/s40880-018-0318-8
- https://cancercommun.biomedcentral.com/articles/10.1186/s40880-018-0323-y
- https://link.springer.com/article/10.1007/s00280-020-04182-1
- https://www.dovepress.com/a-novel-personalized-drug-screening-system-for-platinum-resistant-ovar-peer-reviewed-fulltext-article-CMAR
- https://onlinelibrary.wiley.com/doi/10.1002/cbin.11622
- https://www.cell.com/cancer-cell/fulltext/S1535-6108(20)30413-X
- https://www.sciencedirect.com/science/article/abs/pii/S0304383521000240
Download all of our papers here.
Application in Drug R&D
The high correlation between drug responses of paired MiniPDX® and PDX tumor graft assay, as well as translational data, suggest that MiniPDX® assay can be an effective pre-clinical testing tool in drug R&D.
Applications of MiniPDX in Drug R&D:
| Drug Development Phase | MiniPDX Benefit |
| Lead Optimization | Fast screening of candidate molecules to identify which is best for further resource investment |
| Lead Validation | Post in-vitro studies, MiniPDX screens using PDX cells can identify the best PDX model for further in-vivo validation |
| Pre-clinical Development | MiniPDX Mouse Trial using fresh tumor samples can identify or verify potential clinical indications |
| Clinical Development | MiniPDX + OMICS analysis can reveal biomarkers for patient stratification and trial recruitment strategy |
In addition, by combining MiniPDX® functional testing with proprietary LIDE OncoVee™ K-cell technology , drug development companies can also identify biomarkers that will be useful in patient stratification for clinical trials. LIDE calls this combined (MiniPDX® trial + omics analysis) approach “Functional Diagnosis” and we believe it is the future of drug R&D. Review a case study and learn more about Functional Diagnosis here.